What is SO2 and how does it get in the air?
What is SO2?
EPA’s national ambient air quality standards for SO2 are designed to protect against exposure to the entire group of sulfur oxides (SOx). SO2 is the component of greatest concern and is used as the indicator for the larger group of gaseous sulfur oxides (SOx). Other gaseous SOx (such as SO3) are found in the atmosphere at concentrations much lower than SO2.
Control measures that reduce SO2 can generally be expected to reduce people’s exposures to all gaseous SOx. This may have the important co-benefit of reducing the formation of particulate SOxsuch as fine sulfate particles.
Emissions that lead to high concentrations of SO2 generally also lead to the formation of other SOx. The largest sources of SO2 emissions are from fossil fuel combustion at power plants andother industrial facilities.
How does SO2 get in the air?
The largest source of SO2 in the atmosphere is the burning of fossil fuels by power plants and other industrial facilities. Smaller sources of SO2 emissions include: industrial processes such as extracting metal from ore; natural sources such as volcanoes; and locomotives, ships and other vehicles and heavy equipment that burn fuel with a high sulfur content.
What are the harmful effects of SO2?
SO2 can affect both health and the environment.
What are the health effects of SO2?
Short-term exposures to SO2 can harm the human respiratory system and make breathing difficult. Children, the elderly, and those who suffer from asthma are particularly sensitive to effects of SO2.
SO2 emissions that lead to high concentrations of SO2 in the air generally also lead to the formation of other sulfur oxides (SOx). SOx can react with other compounds in the atmosphere to form small particles. These particles contribute to particulate matter (PM) pollution: particles may penetrate deeply into sensitive parts of the lungs and cause additional health problems.
Learn more about particulate matter
What are the environmental effects of SO2 and other sulfur oxides?
At high concentrations, gaseous SOx can harm trees and plants by damaging foliage and decreasing growth.
SO2 and other sulfur oxides can contribute to acid rain which can harm sensitive ecosystems.
Learn more about acid rain
Visibility
SO2 and other sulfur oxides can react with other compounds in the atmosphere to form fine particles that reduce visibility (haze) in parts of the United States, including many of our treasured national parks and wilderness areas.
Learn more about visibility and regional haze
Deposition of particles can also stain and damage stone and other materials, including culturally important objects such as statues and monuments.
What is being done to reduce SO2 pollution?
EPA’s national and regional rules to reduce emissions of SO2 and pollutants that form sulfur oxides (SOx) will help state and local governments meet the Agency’s national air quality standards.
Learn about how air quality standards help reduce SO2
EPA identifies areas where the air quality does not meet EPA SO2 standards. For these areas, state, local, and tribal governments develop plans to reduce the amount of SO2 in the air.
Learn more about SO2 air quality designations and state implementation plans (SIPs)
What is PM
PM stands for particulate matter (also called particle pollution): the term for a mixture of solid particles and liquid droplets found in the air. Some particles, such as dust, dirt, soot, or smoke, are large or dark enough to be seen with the naked eye. Others are so small they can only be detected using an electron microscope.
Particle pollution includes:
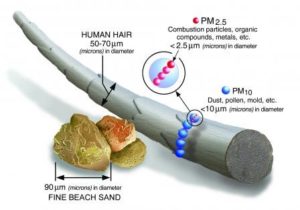
PM10 : inhalable particles, with diameters that are generally 10 micrometers and smaller; and
PM2.5 : fine inhalable particles, with diameters that are generally 2.5 micrometers and smaller.
How small is 2.5 micrometers? Think about a single hair from your head. The average human hair is about 70 micrometers in diameter – making it 30 times larger than the largest fine particle.
Sources of PM
These particles come in many sizes and shapes and can be made up of hundreds of different chemicals.
Some are emitted directly from a source, such as construction sites, unpaved roads, fields, smokestacks or fires.
Most particles form in the atmosphere as a result of complex reactions of chemicals such as sulfur dioxide and nitrogen oxides, which are pollutants emitted from power plants, industries and automobiles.
What are the Harmful Effects of PM?
Particulate matter contains microscopic solids or liquid droplets that are so small that they can be inhaled and cause serious health problems. Particles less than 10 micrometers in diameter pose the greatest problems, because they can get deep into your lungs, and some may even get into your bloodstream.
Fine particles (PM2.5) are the main cause of reduced visibility (haze) in parts of the United States, including many of our treasured national parks and wilderness areas.
Learn more about health and environmental effects
What is Being Done to Reduce Particle Pollution?
EPA regulates inhalable particles. Particles of sand and large dust, which are larger than 10 micrometers, are not regulated by EPA.
EPA’s national and regional rules to reduce emissions of pollutants that form PM will help state and local governments meet the Agency’s national air quality standards. Learn about how air quality standards help reduce PM.
How Can I Reduce My Exposure to PM?
You can use air quality alerts to protect yourself and others when PM reaches harmful levels:
AirNow: Every day the Air Quality Index (AQI) tells you how clean or polluted your outdoor air is, along with associated health effects that may be of concern. The AQI translates air quality data into numbers and colors that help people understand when to take action to protect their health.
Go to About AirNow to learn how you can get AQI notifications.
Also learn how the Air Quality Flag Program can help air agencies, schools, and other community organizations to notify their citizens of harmful conditions and adjust outdoor physical activities as needed.
What is NO2 and how does it get in the air?
Nitrogen Dioxide (NO2) is one of a group of highly reactive gases known as oxides of nitrogen or nitrogen oxides (NOx). Other nitrogen oxides include nitrous acid and nitric acid. NO2 is used as the indicator for the larger group of nitrogen oxides.
NO2 primarily gets in the air from the burning of fuel. NO2 forms from emissions from cars, trucks and buses, power plants, and off-road equipment.
Effects of NO2
Health effects
Breathing air with a high concentration of NO2 can irritate airways in the human respiratory system. Such exposures over short periods can aggravate respiratory diseases, particularly asthma, leading to respiratory symptoms (such as coughing, wheezing or difficulty breathing), hospital admissions and visits to emergency rooms. Longer exposures to elevated concentrations of NO2 may contribute to the development of asthma and potentially increase susceptibility to respiratory infections. People with asthma, as well as children and the elderly are generally at greater risk for the health effects of NO2.
NO2 along with other NOx reacts with other chemicals in the air to form both particulate matter and ozone. Both of these are also harmful when inhaled due to effects on the respiratory system.
Learn more about Particulate Matter and Ozone.
Environmental effects
NO2 and other NOx interact with water, oxygen and other chemicals in the atmosphere to form acid rain. Acid rain harms sensitive ecosystems such as lakes and forests.
The nitrate particles that result from NOx make the air hazy and difficult to see though. This affects the many national parks that we visit for the view.
NOx in the atmosphere contributes to nutrient pollution in coastal waters.
What is being done to reduce NO2 pollution?
EPA’s national and regional rules to reduce emissions of NO2 and NOx will help state and local governments meet the National Ambient Air Quality Standard (NAAQS).
Learn about how air quality standards help reduce NO2
EPA identifies areas where the air quality does not meet the national NO2 standards. For these areas, state, local, and tribal governments develop plans to reduce the amount of NO2 in the air.